Empowering precision drug development
Clinicogenomics data offer a new frontier for insights about potential treatments, target populations and safety profiles.
- Hi everybody, thank you for joining us for this session. We're going to talk about clinical genomics. My name is Dr. Ashley Brenton and I'm the Vice President of Real-World Evidence and Genomics for Optum Life Sciences. I'm thrilled to be joined today by two world-class experts in genomics and its application in patient care and drug development. Dr. Nancy Mendelsohn has been a board-certified clinical geneticists for nearly 30 years caring for adults and children with rare diseases. She currently serves as a senior vice president of Medical Affairs for Genetics and Rare Diseases at UnitedHealth Group. Dr. Mendelsohn, thank you for joining us today. And Dr. Gboyega Adeboyeje is a global value evidence lead for precision medicine at Merck, where he leads Real-World Evidence Strategy Development and Execution to perform translational, clinical and commercial objectives across a broad portfolio of biomarker targets and diagnostic platforms. Dr. Adeboyeje, thank you very much for joining us today. Welcome to both of you.
- Thanks for having us.
- Thank you.
- Now I'm so excited because we're going to spend some time today talking about clinical genomics. We're gonna nerd out. It's going to be a lot of fun and I'm gonna start actually by giving a few definitions. So I think that we're going to talk through some terminology that our viewers might not be familiar with. So people always ask me, what's the difference between genetics and genomics and let's start with DNA. So DNA forms the building blocks of life, really it's the blueprint and our DNA is packaged into chromosomes. These chromosomes are then packaged together into genes and the study of those genes is the field of genetics. When we take all of those genes together and we study their interaction, as well as their interaction with our environment, that is the study of genomics. If we take that one step further and link that genomic information to clinical information, such as disease progression or medication response, that's the field of clinicogenomics. And that's where we're going to focus today. When we discuss DNA, we often talk about something called sequencing, and sequencing is where we read the sequence of the DNA to see where there might be variations. Some of these variations are completely harmless. They determine factors like hair color or eye color, some however can be problematic. They can determine a susceptibility to a disease or a difference in one's ability to process medications. When talking about sequencing, we talk about a couple of different types. The first is germline, that's our normal inherited healthy DNA. And then we also talk about somatic sequencing, that somatic sequencing is the sequencing of cancer DNA. You might not know that often cancer has a different sequence than the healthy host DNA, and often it's those mutations in DNA that allow the cancer to grow and spread. When we talk about genetic testing, many people are familiar with the field of pharmacogenetics and that is simply the genetic variations that affect how we metabolize medication. All right, I think we have all of the definitions and housekeeping out of the way, so we can finally dig in. We know that there has been a ton of progress in genomics over the last 30 years. We went from the human genome project where it took a decade and billions of dollars to come up with one human whole genome sequence, to now where it takes a few days and secretly, it often takes less than that, and less than a thousand dollars for one human genome sequence. We've seen a number of other highlights along the way. So what are some of these? Well, we saw the FDA approval of the first drug in cystic fibrosis that actually covers the underlying cause of the disease. We've seen the development of personalized, targeted treatments for cancer, and we know that this is continuing to develop. It's a very exciting time as we harness this progress to make real changes in precision medicine. So let's talk a little bit about, we've all here in this panel have been in genomics for a long time. So let's talk a little bit about how we got started. I know that my personal story is probably a little bit different. I'm a trained lab scientist, so I'm a molecular biologist. And I started over 20 years ago when we did DNA sequencing all by hand. Sometimes I would get 20 samples extracted a day, and that was really exciting. We would run huge sequencing gels. So these were giant pieces of jello. We'd shoot them with electricity, just like Frankenstein, and that would separate the DNA. It's been really exciting to see all the developments in this space, and I'd love to hear from both of you how you got started. Dr. Mendelsohn, let's start with you. How did you get started in genomics?
- Hi Ashley, thanks, that's a really a great question. So I guess you have to remember that I started a good 10 years before the genome was sequenced, so that's a long time ago, and we had difficulties identifying genes. I went, so that means that when I went to medical school and then I did a pediatric residency and a fellowship in genetics, and I started in the field because I had a personal connection to genetics and genetic disorders. I had a family member that was sick with spinal muscular atrophy and just locating the gene was a big deal. When I think about how far we've come in terms of diagnosis and recognizing SMA even in a prenatal screening. And now that we have a treatment and we're seeing children with SMA who can walk, it's unbelievable. And it's a huge change, things have been very slow and very fast at the same time.
- It's amazing. You were talking a little bit about how it was difficult to even recognize genes for a while. I remember when I came home from college one semester, we had a family friend who was a neurosurgeon and he was in his 90s at the time. And he said, "what classes are you taking?" I said, "I'm taking molecular biology." And he said, "that's the easiest class I ever took." I said, "yeah, they hadn't even deciphered the structure of DNA when you were in molecular biology." So it's funny to see how far it's come. And now Dr. Adeboyeje how about you, how did you get started in genomics?
- Yeah, thanks for that question. But I think unlike Dr. Mendelsohn my involvement with the field has not been that long. I joined the field a few years ago, 2018. And when I joined Merck, I think the time was the time where you have this increasing affordability of cancer sequence examination and also the explosion in the number of biomarker defined indications approved by the FDA. The other thing is also that you have new paradigms of drug development to tumor-agnostic indications where the indication is not based on the tissue of origin, it's based on the presence or absence of a certain genomic target. And the other thing is also the challenges of demonstrating the value and affordability for this biomarker defined indications that requires some sort of like sequencing for patient selection, so it's an exciting time in the field and some of the advances we've made in this field in the last couple of years has been really dramatic when you see the magnitude of clinical benefits for a certain subset of patients that derive benefit from those indications. And also that in the last couple of years, I think the scientific community is increasing understanding that tumors that we used to think of as a single disease entity, are actually, collections of smaller subsets of molecular disease entities. And I think that has really, really changed the part on drug development as well. So the challenge now is moving all of these discoveries and approvals from the clinical realm into the background where it's not just research or in academic centers, but in routine care as well. And I think that's the promise of the field, as well.
- It is, it's been really exciting progress. So you said that you transitioned into genomics several years ago and you were a primary care physician before that, is that correct?
- Yes, I was a primary care physician when I left clinical practice. I took some training in clinical decision science or what you call Decision Theory, which is how do you quantitatively optimize decision-making in clinical care, and I think prime application of that field of science is diagnostic testing and also how you decide the optimal pathway from treating based on biomarker indication to clinical outcomes. So it's been, I think from that perspective, it's been quite an exciting journey in terms of my career and also the opportunity to contribute to the field in terms of helping to optimize the value and also the place of sequencing in patient care.
- Very exciting, now, Dr. Mendelsohn, as you've been in this field for a while, how have you seen advances in technology reflected in your own work?
- Well, there've been a lot of changes, the ability to treat people, early on what a clinical geneticists did was look at somebody from a dysmorphology perspective so recognize what the syndrome was. And there wasn't a lot of treatment we could do, we could support people and we could help take care of them from a symptomatic perspective. The biggest difference is now in addition to being able to recognize people, we can do a much more accurate molecular diagnosis for their disorder which is very reassuring to families to have an answer for a child or a loved one who very sick. And now in the more recent years we actually have therapies. So you mentioned cystic fibrosis. It's a great example of personalized medicine because we have medications that are specific for the particular mutation and that CFTR gene, medications that address the structural malformation of the gene or medications that address a gating mutation. It's very exciting. And most recently we have true gene therapies. We have therapies that can correct the underlying gene mutation in the cells and allow people then to have a relative curative state to their disease.
- That's amazing, we're now talking about curing, right? As opposed to just treating. Now, this might come out of left field, do you have any, I'm gonna ask both of you, do you have any predictions or any best guesses for what you think the future holds in precision medicine? Dr. Adeboyeje, what do you think?
- Yeah, I wouldn't say this is a prediction, I think is more of an evolution, right? But I think we're getting to the stage where if a patient comes in with a new diagnosis of cancer, the optimal step would be to do some upfront sequencing to characterize the underlying biology of that tumor and be able to select is that approved therapies or look for opportunities to enroll that patient into clinical trials, so it's something that's been developed for the last, over the last couple of years. So it's not so much of a jump from what we currently have. Right now in lung cancer there are several guidelines and specialty societies which recommend sequencing patients up front especially patients with advanced disease. But I think that paradigm would also translate into other cancer areas as well and specialties as well. We're seeing the same thing in prostate cancer right now. We're seeing the same thing in breast cancer where it's really important because of the dramatic benefit that some certain patients derive from newly approved drugs to really explore if that patient can otherwise benefit from an approved therapy. So I think from that in the next, I would say, short to medium term, I'm talking three to five years, that would be standard of care of advancing with broad comprehensive genomic profiling to really obtain a lot of detail on what that patient might potentially respond to and post through that pathway. And also the fact that we are beginning to explore, in greater detail the use of sequencing in understanding the mechanisms of resistance. And that can also, the explosion in the approval of targeted therapies that can help inform whether you can rechallenge a patient with a previously, therapy they previously received or you have to switch to a new regimen. So it's an exciting time, and I think patients are beginning to see a lot of that benefit in dramatic increases in overall survival as well. So that's my, I would say that's my take on where the field is going.
- Excellent, Dr. Mendelsohn what about you, any predictions?
- I don't know if they're predictions as much as hopes, right? I mean, I would like to see us get to a point of making diagnoses more rapidly, trying to prevent the diagnostic odyssey pathway, getting genetic testing into the hands of our primary care physicians so that the testing is done sooner and we can reduce the morbidity and the mortality for people with rare diseases. I think the treatment will continue to evolve and become simpler, be less onerous, have less complications rapidly, that's the side that is quickly making advances. And it's very exciting. Our challenges are gonna be around adoption and implementation, right? Trying to teach people to partner with people and to have these tools in the hands of more clinicians.
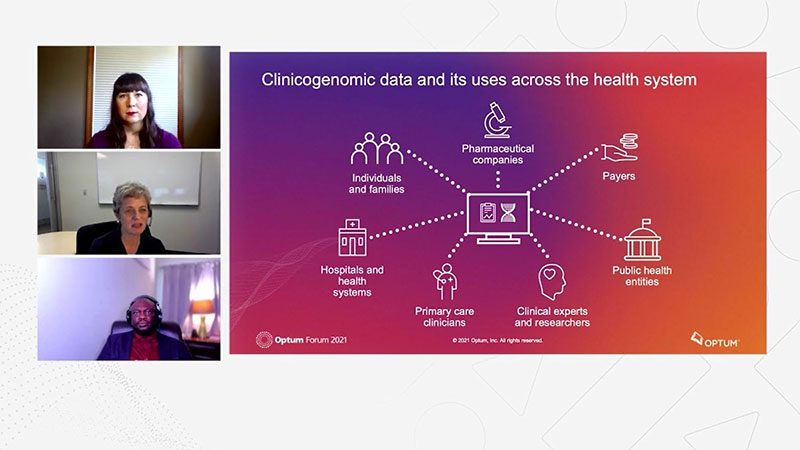
- Absolutely, and I think when we talk about precision medicine, there are a number of stakeholders as well. So that entire healthcare ecosystem, many different stakeholders, everybody from let's say, pharmaceutical companies to clinicians, public health entities, the clinical scientists, the healthcare systems, Dr. Adeboyeje, could you speak a little bit about how pharmaceutical companies and researchers are using clinicogenomic data in drug discovery and development?
- Yes, that's a broad question in the sense that you will think of the spectrum of drug discovery from the translational discovery stage to clinical trials and also from what Dr. Mendelsohn said about getting the the diagnostic assays and biomarker information into the hands of clinicians at the point of care, I see a lot of possibilities here. One characterizing the phenotype clinical presentation of this molecularly defined subsets of cancer patients, and also trying to characterize the natural history of disease, the unmet need in terms of either in their resource use and all of that. And to also help inform clinical trial design from looking at prevalence and the distribution of these patients, any differences or differential distributions of this mutation across various subgroups, racial, ethnic, and also geographic regions. And the challenge is not so much about getting a drug approved or a companion diagnostic approved, but how does it get to the point of care that it becomes routinely used? So you have the optimal use of that drug by the right patient at the right time. So clinicogenomics data is something that can inform every aspect and every stage of drug development from discovery, translational stage through clinical development and also the end stage of affordability and reimbursement and access as well. So it's critically important and when you see the proportion of bio backup based or defined trials on clinicaltrials.gov, you begin to appreciate the substantial unmet need for this kind of a high quality, research ready, regulatory great data to form strategic objectives, related to drug development, commercialization and action.
- You touched briefly on something that I think is really important for viewers to think about, which is the diversity of these clinicogenomic data sets. So clinicogenomic data sets are very difficult to build. They're expensive, it's slow. Many of the commercially available biobanks were developed with government funding. And what we tend to see is, I like to call it the homogeneity problem. We see that many of these individuals are Caucasian, they're of European descent, and these biobanks form the basis of a lot of what we know about disease progression, right? It starts with the data. We use these for these big genome-wide association studies to understand disease progression, to identify biomarkers. And so we see that lack of diversity causing problems, not just in our understanding of patient natural histories, but also into drug discovery and development, into clinical trials, and then into making sure that medications are safe and effective for everyone. So I think that's an important topic that we should continue to focus on in the field is how we increase the diversity and representative nature of these clinicogenomic data sets.
- Yeah, and I think you raise a very important point, recently everybody is aware that health equity, and disparities research has become front and center of our conversation in health care and how to ensure more equitable distribution of healthcare resources. I think precision medicine and clinicogenomics data can help begin to address some of those unmet need in terms of how do you target patients for enrollment in clinical trials and the kind of information clinical trial evidence you produce to make sure it applies to various patient populations in a way that's culturally sensitive and also equitable as well, so it's really important from that perspective as well.
- Absolutely, absolutely. Something we will certainly continue to work on. I know that's something my team is working hard on. Now, Dr. Mendelsohn, I'm gonna turn to you, certainly you're a stakeholder in clinicogenomic data use and precision medicine focusing in rare disease. Can you touch a little bit on, sort of your experience here? I know we had a really interesting conversation the other day about newborn screening, and I was wondering if you could share some of your insights for our audience.
- Yeah, I'd be happy to, I mean, one thing just thinking back about the economic burden of rare disease is I'm sure most people that are listening to this conversation know about The EveryLife Foundation and Lewin study that looked at a comprehensive assessment of the economic burden of rare disease in the United States in 2019. And they looked at 379 rare diseases and noted a prevalence in 15.5 million people, with an economic cost of $966 billion. I mean, that half of that is medical direct cost and the other half is indirect. And that means that there is a lot of cost in the families that are taking care of these individuals. So the parents missing work, loved ones missing work and that kind of thing. So I didn't wanna skip over that. I wanted to make sure I mentioned it today. In terms of newborn screening and what we've learned and how about rare diseases. I believe that as we begin to treat people, and as we begin to recognize the diseases, our understanding of the phenotype, the clinical expression of the disease, it broadens. Let me give you an example of that if I may, when I was in school and a resident Pompe disease was considered to be a disease of cardiomyopathy where newborns died and the babies, they were born, they had heart disease, and then their peripheral muscles, they had peripheral myopathy as well. And they just, we had no treatment. And the gene was found and fortunately, we have enzyme replacement therapy now, and we began to treat these children and they survived. And now it's on many states' newborn screening. It's wonderful. And as we've begun to treat people, we learned that actually there are adults that have late onset Pompe disease, now considered to be a similar but very distinct disease. And they don't have any heart disease. They have a peripheral myopathy, but in general their hearts are just fine. So the reason I explain this is that as we recognize what the disease is, we can then begin to understand the breadth of the phenotype, all the different ways the disease can present, then we can begin to look for more biomarkers. We can begin to understand better how to treat people and fortunately develop more treatments. So I hope that answers the question you wanted and is helpful.
- Yes, thank you for those insights. Another stakeholder is of course payers, right? Dr. Mendelsohn, could you talk a little bit about how payers use clinicogenomic data to support population health initiatives?
- Sure, it's not a one and done, it's a complex field I would say, the payers are looking for clinical outcomes. We wanna understand that the treatment that is being made available to people with rare disorders is helping them. And we want to be able to measure that in concert with their clinicians, their expert doctors. We want to be able to prove to the people that employ these individuals that are the employers of the parents, that these expensive medications actually are saving lives and are creating better outcomes. And it's really hard because we don't have standard outcomes across these rare diseases because they're just that rare. And we know so little about them as things change over time. So we use the clinicogenomic data to look at what is the mutation? What is the disease? How are the children and the adults doing with these treatments? How are the outcomes? And are they supporting the families? Are we getting people back to work? Are children able to go to school? What's the whole picture?
- Absolutely, and I think you touched on something really important here, which is that rare disease doesn't just affect the patient, but also their caretakers and the entire family network. And as we think about clinicogenomic data, I think that it's important for us to look into that as well, right? So we look at the patient, but we look beyond the patient at everyone else who's affected as well. And those could be some of the outcomes that we measure.
- Right, you're absolutely right. I mean, we know that parents and loved ones of someone with a rare disease tend to put off their own care and they don't do their own regular routine screening and medical care. And so we want to be able to support them so that they can take care of themselves as well.
- Yeah, to add to what you've both expressed, I think that's also one of the biggest challenges of clinicogenomics data right now, to be able to link downstream clinical outcomes to treating based on biomarker testing and all of that. And Dr. Mendelsohn mentioned rare disease, but it also applies to oncology and other areas as well. And I think the industry is beginning to recognize the need for such launched-to-know high quality datasets, to be able to demonstrate values from a lot of dimensions, clinical dimensions and also economic dimensions of value as well, making sure that where it should demonstrate actual clinical outcomes to help payers make some of these decisions as well.
- And that of course is one of the large challenges, is how do we one, to find value and two, how do we demonstrate value? I know that that's always the challenge for all of you, all of the stakeholders here, right? In this community ecosystem that we have.
- Right, then I hesitate to say but also head to head drug studies, right? Like how do we know what is the right time to start that gene therapy, when do we start? Is it before there are any symptoms? I would hope so, but when are the symptoms gonna start? And that if you have a lethal newborn disease, that's easy, but if you have a slowly progressive disease, that can be much harder to determine.
- And how do we know when to order the genetic testing that could inform some of these decisions as well? All right, so I know that we on the panel are convinced that there is amazing potential with clinicogenomic data. And I am sure all of our audience is now convinced as well, but of course there are a number of hurdles, right? So a few of these, we touched a little bit on the lack of diversity in clinicogenomic data sets. A lot of interest has been in consumer genomics. So that's a lot of the data that's available. Some of the genomic data is inconsistent. It might not be as deep as we would like. So instead of talking always about whole genome sequencing and comparing it to other whole genome sequencing, the quality could be different, or we could be comparing single gene sequencing or panels to whole genome sequencing. So I think that lack of consistency and quality can be detrimental. And then of course, we've all talked about how difficult it can be to find datasets that link that high quality genomic sequencing to robust longitudinal clinical information. Of course, Dr. Mendelsohn you touched on how do we measure outcomes? And we know that building these data sets is expensive and resource intensive. So those are a few of the first challenges that I see, Dr. Adeboyeje would you like to touch on any other hurdles that you see in this space?
- Yeah, you asked about value demonstration and how do we define value? I think the first question I would ask is that, whose value and who's the audience for that value demonstration. We talked about payers in terms of what kinds of value and outcomes they consider important in making some of their own decisions. But we also have to think about the physician and the patient aspects of that as well. And it could range from the standard clinical utility, clinical variability to make sure that that biomarker status define a well-defined patient pattern of presentation or what you call clinical phenotype, but also that in the context of emerging drug development paradigms and treatment pathways where you have this tumor-agnostic indications for cancer types that are not high prevalence, you have to come up with new ways to measure the value of that intervention. And I will give you an example regarding that, say you have a tumor-agnostic indication, right? And the prevalence of that indication is about, let's say 2% or 5%, the choice becomes should you sequence with the hotspots panel or a comprehensive genomic panel. And when you sequence with a very broad panel, the benefits do not accrue just to that tumor type alone, you can also potentially determine the biomarker status of several of the FDA approved medications as well. So I think there is need for new value assessment paradigms, new methods to assess the value of clinicogenomics indications, drug indications, and also how we assess whose value, whose outcomes are we looking to examine and address as well. The other thing is also that right now we have different approaches to development of assays and platforms, right? The various options available on the market do not always address the same thing or even define the same biomarker status, there is a need for that. There's also a need for outside of clinical trials to have high quality information to be able to address some of these value assessments as well. And also when you have dramatic clinical benefits from certain biomarker defined indications, right? How do we quantify that? Nowadays we're beginning to think of a curative intent for certain tumors that five, 10 years ago we thought are incurable so that's something that from a, I would say in methodology and clinical perspective and also from a policy perspective, would need to be addressed as well.
- Excellent, thank you, and you touched a little bit on the need for standardization, who do you think leads that effort?
- I think there's a role for regulators to play in that efforts to standardize biomarker definitions and also platforms and assays that we use to identify this or assess this biomarkers. There is also a role for cross, I will say, cross-stakeholder, multiple stakeholder collaboration in the field from a variety of perspectives, test developers, drug developers and the payers as well to come together to begin to address some of the discrepancies and the variation in how you define certain biomarkers and also the developments of these assays. But I think by and large, most of the responsibility for that should fall to the regulators as well.
- So, Dr. Mendelsohn we've talked a little bit about how many of these datasets lack representation, or perhaps there's inconsistent depth or quality of the data when you're working with rare disease, where there are so few individuals represented for each condition, how does this inconsistency affect your work?
- Yeah, it creates all kinds of challenges. I mean, just the concept of clinical utility and being able to prove that a medication or a treatment is appropriate for a rare disease can be difficult. The inability, as we mentioned, to do head-to-head studies or create analytic validity of the data is really a challenge, that being said, I do think there are some underlying commonalities to rare diseases and we have opportunities to learn from one disease set and apply to another. And I'm hoping that's where the field of clinicogenomics can assist us. I also think we have a huge opportunity in terms of access and diversity and inclusion. So we have not done a great job historically of ensuring that medications are equally accessible across all populations, and we have work to do there.
- Absolutely, we do. We've certainly come a long way, but we have a long way to go. I am thrilled to see that there are so many good people working in the space and that we're starting to talk about many of these issues that do exist. I'd like to thank you both for being here with me today. And of course our audience for watching. Before we wrap up I would like to mention that if you have any questions you can submit a question using the link below the video. So thank you all for joining me today.
- Thank you for having us.
What Clinicogenomics Means for Drug Development
Experts from Optum, UnitedHealth Group and Merck discuss clinicogenomics and new insights for potential treatments, target populations and safety profiles. Explore how linking phenotypic and genomic or other -omic data helps to assess the full view of the patient journey.
Learn more about Optum Clinicogenomics.
Visit the Optum Life Sciences home page.
Fuel your next discovery
Streamline the drug research and development process through discovery of high-value biomarkers, the genetic landscape and barriers to access.
Related content

Clinicogenomics: new, linked data is advancing research

DNA, Personalized Medicine and…Zebras?
Nearly 30 million Americans live with a rare disease. Hear how genomics are shaping how we treat everything from ultra-rare diseases to the most prevalent cancers.



